Agdia has released a rapid isothermal molecular assay for the detection of Botrytis cinerea on their AmplifyRP® XRT platform.
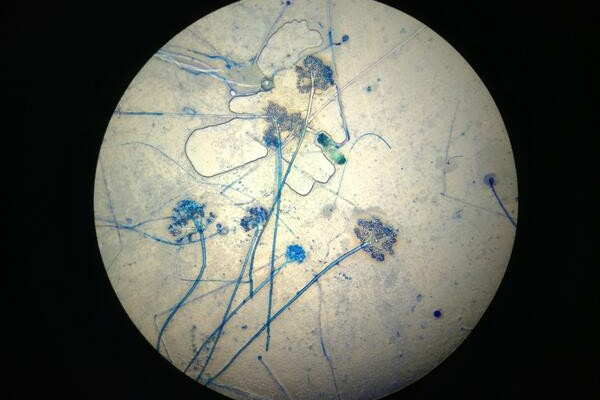 Figure 1. Microscopic view of conidia of Botrytis cinerea.
Figure 1. Microscopic view of conidia of Botrytis cinerea.
Botrytis cinerea is an ascomycete fungus and is considered one of the most important and destructive phytopathogens on the planet. Botrytis cinerea and its numerous pathosystems have been studied extensively and are considered models for molecular interactions between plants and pathogens. This generalist necrotroph is capable of causing severe diseases in more than 500 species of economically important plants. Hosts include vegetable crops such as cabbage, cucumber, lettuce, onion, potato, squash, and tomato; fruit crops such as grape, peach, raspberry, and strawberry; floral and ornamental crops such as aster, begonia, chrysanthemum, geranium, marigold, peony and rose; and medicinal and oil crops such as cannabis and sunflower. Worldwide, it is estimated that Botrytis spp. cause tens of billions of dollars in crop losses annually, in the field and post-harvest.
 Figure 2. Gray mold disease on grape cluster
Figure 2. Gray mold disease on grape cluster
The genus Botrytis was characterized in 1729, making it one of the first genera of fungi to be established. The name Botrytis cinerea was first used in 1771 to describe the species. The word botrys is Greek for “a cluster of grapes” and is combined with the Latin suffix -itis meaning disease to form Botrytis. The specific epithet cinerea is Latin for “resembling ashes” or “ash-colored.” Collectively, the species name can be translated as “a grape-like disease that looks like ashes.” The “cluster of grapes” designation refers to the arrangement of asexual spores known as conidia on fruiting structures when viewed microscopically (Figure 1.). The description of ‘ash-colored” applies to the considerable amounts of fuzzy gray mycelia produced during the necrotrophic phase of gray mold infections (Figure 2.).
In addition to gray mold, B. cinerea causes blossom and petal blight (Figure 3.), blossom end rot, bud rot, bulb rot, fruit and head rots (Figure 4.), leaf spots, stem cankers (Figure 5.) and damping off in seedlings, pre- and post-emergence.
Therefore, infections take multiple tracks, and symptoms vary according to the host, tissue infected, and environmental conditions. Indeed, the success of B. cinerea as a plant pathogen is due to its considerable host range, high reproductive capacity, numerous infection strategies, and ability to survive as sclerotia in soil and crop debris for extended periods of time. Furthermore, field infections can remain asymptomatic as the pathogen lies dormant within plant tissue or putatively survives as a biotroph. Biochemical changes within the senescing tissue can affect the transition to an aggressive necrotrophic phase in post-harvest storage and on supermarket shelves (Figure 6.)
 Figure 3. Petal blight on rose flowers
Figure 3. Petal blight on rose flowers
Botrytis cinerea is typically an airborne pathogen. Infected plants produce copious amounts of asexual spores known as conidia, which function as the primary means of dissemination. Conidia are spread to healthy plants via wind currents and splashing water. Upon arrival, these spores germinate to form infection hyphae known as germ tubes, which actively penetrate plant tissue. Furthermore, damaged tissue and natural openings function as infection courts. During the necrotrophic phase of infection, B. cinerea produces effectors and toxins, manipulating plant immune responses and killing cells, respectively. As the advancing fungal mycelium colonizes necrotic tissue, it feeds on cellular contents released as a result of its activity, hence the necrotroph designation. Diseases caused by B. cinerea are polycyclic, and conidial sporulation occurs throughout the life of the plant tissue infected. Hardened masses of mycelium, known as sclerotia, form in dead plant tissue and function as survival structures and inoculum for future infections. Sclerotia can germinate directly to produce infectious mycelium and conidia subsequently (Figure 7.).
 Figure 4. Head rot on cabbage.
Figure 4. Head rot on cabbage.
Management of diseases caused by B. cinerea has relied heavily on the use of synthetic fungicides, historically. Indeed, it is estimated that the chemical management of B. cinerea accounts for 8% of the global fungicide market. Nevertheless, this pathogen has proven resilient via its ability to develop resistance to multiple fungicide modes of action across diverse cropping systems; resistance runs the gamut from reduced efficacy to complete failure in the field. The responsible rotation and combination of fungicide products are paramount to maintaining efficacy against B. cinerea. Unfortunately, robust genetic resistance to B. cinerea has proven to be elusive, and selective breeding is ongoing.
 Figure 5. Stem canker infection at site of pruning wound on tomato
Figure 5. Stem canker infection at site of pruning wound on tomato
Successful management of an aggressive necrotroph such as B. cinerea is dynamic and multi-faceted, utilizing clean propagative materials, early detection, responsible fungicide rotation, irrigation management, proper plant spacing, ventilation, and sanitation. Disease mitigation is complicated further due to this pathogen’s wide host range, numerous infection strategies, and the diverse cropping systems affected. For example, most field crops can be treated with fungicides as needed, and human interaction with plants is minimal. However, environmental variables such as temperature, humidity, and precipitation are unmanageable under field conditions.
 Figure 6. Gray mold infection on strawberry fruit
Figure 6. Gray mold infection on strawberry fruit
Indoor agriculture minimizes environmental variables but is highly susceptible to human-assisted spread of pathogens via cultivation and propagation. Furthermore, medicinal crops such as cannabis are subject to strict regulations regarding synthetic pesticides, and there is a paucity of effective approved biological pesticides on the market. Collectively, these make B. cinerea a formidable adversary in civilization’s ongoing struggle to manage phytopathogens.
 Figure 7. Sclerotia (upper right), mycelia (upper left), and conidia (lower right) of B. cinerea on green chili pepper fruit
Figure 7. Sclerotia (upper right), mycelia (upper left), and conidia (lower right) of B. cinerea on green chili pepper fruit
Early and accurate pathogen diagnosis is paramount to the success of any disease management program. Agdia’s new AmplifyRP® XRT assay for the detection of Botrytis cinerea provides growers from multiple cropping systems with varying levels of expertise with a powerful diagnostic tool. AmplifyRP® XRT technology promotes the rapid amplification and detection of nucleic acid targets, DNA, or RNA while maintaining a single operating temperature of 42oC. The AmplifyRP® XRT products achieve target sensitivity and specificity comparable to qPCR while having clear advantages over the lab-based technology. AmplifyRP® XRT products do not require a nucleic acid purification step; crude sample extracts are prepared using a simple extraction buffer and tested directly. When paired with Agdia’s AmpliFire® isothermal fluorometer, the XRT system is a rapid, user-friendly tool that can be implemented in the field or the lab by personnel with limited experience in molecular diagnostics (Figure 8.).
plifyRP® XRT assay expands their catalog to 26 plant pathogen products on this platform.
As a leading provider of diagnostic solutions for agriculture, Agdia, Inc. has been serving plant breeders, propagators, growers, universities, regulatory organizations, and private testing laboratories since 1981. The company offers a comprehensive portfolio of validated, easy-to-use diagnostics for identifying plant pathogens, plant hormones, and transgenic traits and comprehensive customer support on all products. Furthermore, Agdia operates an ISO-accredited, in-house testing services laboratory.
For more information:
Agd ia
ia
52642 County Road 1
Elkhart, IN 46514
Tel.: 1-574-264-2615
Fax: 1-574-264-2153
[email protected]
www.agdia.com
